Memic Innovative Surgery announced today that it closed a $96 million Series D financing round.
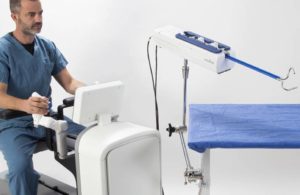
Tel Aviv, Israel-based Memic develops Hominis, the first FDA-approved robotically-assisted surgical device for performing transvaginal hysterectomy, with indication for performing benign hysterectomy with salpingo-oophorectomy. The FDA approved the Hominis system last month.
Hominis is designed to remove the uterus using minimally invasive surgical instruments inserted through the vagina, as well as a video camera inserted laparoscopically through a small incision on the abdomen. The transvaginal approach requires fewer incisions on the abdomen compared to the traditional laparoscopic hysterectomy.
According to a news release, Memic plans to use funding to support the commercialization of the Hominis platform in the U.S. and potentially elsewhere as marketing and sales efforts in other countries are expected to be expanded.
Further uses of the funds include the company’s continued research and development efforts and the expansion of Memic’s portfolio of products, as well as a manufacturing scale-up and improved customer support and training.
Peregrine Ventures and Ceros led the financing round with participation from Ourcrowd and Accelmed.
“The Hominis system represents a significant advancement in the growing multi-billion-dollar robotic surgery market,” Memic co-founder & CEO Dvir Cohen said in the release. “This financing positions us to accelerate our commercialization efforts and bring Hominis to both surgeons and patients in the months ahead.”

