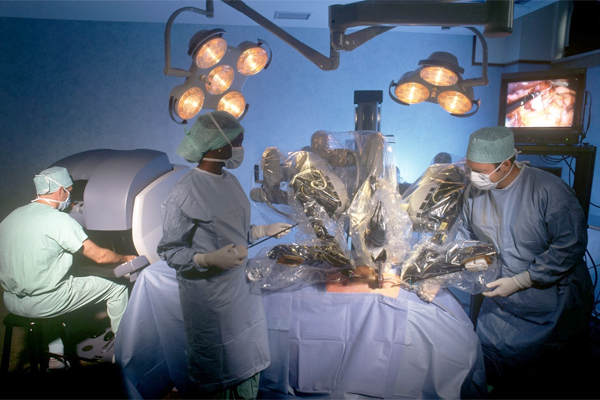
The FDA plans to hold a public workshop on robot-assisted surgical devices to get feedback on how such products should be regulated in the future.
The workshop, which will be webcast, is slated for July 27-28 at the agency’s White Oak campus in Silver Spring, Md. Those interested in attending in person or viewing the webcast must register online by July 17. The deadline for submitting written comments is August 26.
“There are several clinical and scientific challenges associated with regulation of RAS devices, such as appropriate non-clinical and clinical evaluation of RAS devices, use of third-party surgical instruments with legally marketed RAS devices, and clinical-training programs,” the federal safety watchdog said.
“This workshop seeks to involve industry and academia in addressing these challenges in the development of RAS devices to ensure that there is a reasonable assurance of safety and effectiveness for RAS devices while promoting innovation in a rapidly-developing field. By bringing together relevant stakeholders including scientists, patient advocates, clinicians, researchers, industry representatives, and regulators, we hope to facilitate the improvement of this evolving product area,” the agency added.
The workshop will address a number of topics including the FDA’s role in device-training programs, preclinical testing methods, patient-outcome metrics, and the benefits of RAS devices over traditional surgical procedures.