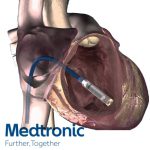
Medtronic (NYSE:MDT) said today it won FDA premarket approval for its Micra transcatheter pacing system, which it claims is the world’s smallest pacemaker.
The Micra transcatheter pacing system, which at 1/10th the size of a conventional pacemaker is roughly the size of a large vitamin, is designed to be implanted via catheter in the right ventricle to deliver single-chamber pacing, Medtronic said. The Micra device has an estimated 12-year battery life and is approved as safe for full-body MRI scans, the company said.
Micra is cleared with indications for patients with a heart arrhythmia called atrial fibrillation or those who have other dangerous arrhythmias, such as bradycardia-tachycardia syndrome, according to an FDA release.
“For many years we’ve been hopeful that a transcatheter pacing solution – with a safety and effectiveness profile on par with conventional devices – would become available, and today Micra has achieved this milestone. In the clinical trial, the Micra was successfully implanted in nearly all patients, and met its safety and effectiveness endpoints by wide margins. This gives us great confidence that this miniaturized device will bring patients the most advanced pacing technology, combined with the less-invasive nature of the new technology,” Micra TPS trial principal investigator Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center said in a press release.
The Micra is the 1st miniaturized pacer to win FDA approval, the Fridley, Minn.-based company said.
The device is designed to be attached to the heart with small tines and delivers electrical pulses to pace the heart through an electrode at the end of the device, and does not require a surgical “pocket” under the skin or leads.
“Dating back to the development of the 1st external battery operated pacemaker more than 60 years ago, Medtronic has a long history of collaborating with clinicians to better understand the needs of patients, and then innovating new products to meet those needs. We are thrilled to be the 1st to introduce a transcatheter pacemaker to patients in the U.S., and we’re looking forward to working with physicians and educating implanters to extend the positive results of our global clinical trial experience to even more patients,” Medtronic cardiac rhythm and heart failure biz prez Dr. John Liddicoat said in prepared remarks.
Medtronic said the Micra TPS is the only transcatheter pacing system to be approved for 1.5 and 3 tesla full-body magnetic resonance imaging scans.
“As the 1st leadless pacemaker, Micra offers a new option for patients considering a single chamber pacemaker device, which may help prevent problems associated with the wired leads,” FDA Center for Devices and Radiological Health Office of Device Evaluation acting director Dr. William Maisel said in an FDA release.
Last November, Medtronic said its micro-sized leadless pacemaker met its primary safety and effectiveness endpoints with “wide margins.” Results from the trial were presented at the American Heart Association’s scientific sessions meeting this week and published in the New England Journal of Medicine, Medtronic said.
Medtronic said that 99.2% of patients in the trial were successfully implanted with the device. A total 96% of 725 patients experienced no major complications, which Medtronic said is 51% less than is seen in patients with conventional pacing systems.
Cardiac injuries occurred in 1.6% of patients, complications at the groin site in 0.7% and pacing issues in 0.3%. Medtronic said there were no dislodgments, no systemic infections and a 0.4% rate of system revisions.
Last April, Medtronic said it won CE Mark approval in the European Union for the Micra, the company said today, touting it as the world’s smallest pacemaker.
The CE Mark was granted based on 3-month results from the 1st 60 patients enrolled in Medtronic’s Micra TPS trial, the company said.