The surgical robotics space saw a major shakeup last year.

Hani Abouhalka, Johnson & Johnson’s company group chair for Robotics & Digital, said those words at our DeviceTalks West event in California in October.
It pretty much sums up the situation for medtech companies large and small that sought to compete against Intuitive in the soft-tissue surgical robotics space. Despite the increased competition, Intuitive remains the undisputed leader. It’s even moving forward on a next-gen da Vinci robot.
For its part, Johnson & Johnson is sticking with plans to develop its Ottava surgical robot. J&J now plans to submit Ottava for FDA investigational device exemption (IDE) in the second half of 2024 in order to start clinical trials.
Even though it is sticking with robotic surgery, J&J laid off employees in the field in 2023. Other companies, including Vicarious Surgical, have also cut jobs.
Meanwhile, Titan Medical has mostly wound down. And Siemens Healthineers said last year that it would discontinue the use of its Corindus surgical robotics for cardiology procedures.
(We’ll have plenty of surgical robotics presentations at our DeviceTalks Boston event, May 1–2. Register here.)
Here are 10 surgical robotics companies that continue to attract attention from MassDevice’s editors and audience:
| Company | Robotic system(s) |
| Intuitive Surgical | Da Vinci 5, Da Vinci Xi, Da Vinci X, Da Vinci SP, Ion |
| Asensus Surgical | Senhance, Luna |
| CMR Surgical | Versius |
| Medtronic | Hugo, Mazor |
| Johnson & Johnson | Monarch, Velys, Ottava |
| Stryker | Mako |
| Moon Surgical | Maestro |
| Stereotaxis | Genesis RMN, Vdrive, Niobe |
| Distalmotion | Dexter |
| Noah Medical | Galaxy |
And here is more about the 10 companies:
Intuitive Surgical: Reaping the benefits of being a pioneer

Since its founding in the 1990s, the Sunnyvale, California–based company has placed more than 8,000 multiport and single-port systems around the globe and racked up more than 14 million procedures, including approximately 2.3 million just last year.
“I do see us as the leader, as the pioneer,” Intuitive President Dave Rosa said during our DeviceTalks West conference in Santa Clara, California, in October 2023.
Promoted to the position of president in May 2023, the 27-year Intuitive veteran offered his perspective on perseverance and shared advice for medical device developers: “Competitors and the growing field, it’s so exciting [because] that validates the … blood, sweat and tears that our team has put in to forge this field and show that there is benefit with robotics. We can do things better, not only bringing more minimally invasive surgery to the world, but doing it with better options than laparoscopy could. … And now we have big and small companies coming in and saying, ‘We can make a difference too.’”
In January, Intuitive announced that it has submitted for FDA 510(k) clearance of its next-generation da Vinci 5 multiport surgical robot. Company officials hope for a launch this year.
The da Vinci 5 will join Intuitive’s existing da Vinci robotic surgical system portfolio, which includes the multiport X and Xi systems and the single-port SP. There is also Ion, Intuitive’s robotic-assisted platform for minimally invasive biopsy in the lung.
In a separate DeviceTalks podcast interview, Rosa identified two technological opportunities that he thinks are going to advance surgical robotics and minimally invasive surgery in a major way: improved visualization for surgeons and focal therapy. The technologies have Rosa thinking of Intuitive as a minimally invasive care company.
Additional Intuitive coverage:
The secret behind Intuitive’s surgical robotics success: Intuitive Surgical Chief Medical Officer Myriam Curet shares how Intuitive has built an ecosystem of success around data and collaboration to support the adoption and use of the technology.
How surgical robotics leader Intuitive is growing in China: It’s the second-largest procedure market for Intuitive Surgical‘s da Vinci system and one of its fastest-growing markets.
Intuitive leaders weigh in on GLP-1 drugs and surgical robotics procedures: Company executives say they see benefits to their business from GLP-1 weight loss drugs, even if investors aren’t quite so sure.
Asensus Surgical: Building a next-gen system that can last
Seven years after its Senhance robotic surgery system won FDA clearance, Asensus Surgical has unveiled its next-gen Luna platform. The company struck a deal with Flex to manufacture Luna and is seeking an FDA clearance in 2025.
During DeviceTalks West 2023, Asensus R&D VP Dustin Vaughan described Luna’s improvements, including a 30% smaller footprint; fully articulated, reusable TrueWrist 5 mm instruments; and a unique surgeon console and ergonomic benefits.
Asensus Surgical recently showed off Luna to surgeons. Here’s a video:
The years of development behind Luna also included the creation of an instrument drive system, a desktop test system to enable more instrument innovation.
According to Vaughan, one of the greatest challenges to overcome when designing the system was to ensure that the platform could support the layering of new, innovative tools and software not just for the next year but for the next decade.
“We’ve seen how challenging this space is to get the capital equipment,” Vaughan said. “As passionate as I am personally about robotics, it doesn’t change the fact that this will be the same system for many, many years. … You’ve got to get this right, because you’re going to look at it and develop on it for a very long time.”
CMR Surgical: A British Invasion for surgical robotics?
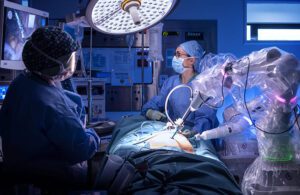
- Bringing on J&J veteran Supratim Bose as its CEO;
- The development of a next-gen system and new products;
- A reorganization to be more focused on the commercialization of the existing system, including the hiring of Johnson & Johnson and Smith+Nephew veteran Massimiliano Colella as chief commercialization officer;
- Continuation of a targeted commercial collaboration with J&’s Ethicon business, with J&J saying in a shared statement that it “underscores Ethicon’s commitment to providing healthcare partners with best-in-class surgical solutions that focus on the unique needs of every patient;”
- Opening a roughly 75,000-square-foot manufacturing facility in Cambridgeshire, U.K. to support expansion;
- Surpassed the company’s fundraising goals with a new $165 million (£133 million) round.
“I think it has been a great year of trying to do what is good for the company,” Bose said in a recent interview with MassDevice. (Hear more from Bose, co-founder and CMO Mark Slack and co-founder and CTO Luke Hares at DeviceTalks Boston, May 1–2, 2024. Register here.)
In 2023, CMR Surgical’s installed base grew 50% to 160, and annual surgical procedure numbers grew 60% to 17,000. Bose ticked through the advantages of Versius, including a smaller mobile design that allows it to be wheeled around health facilities, an open console that enables surgeons to guide their operating room staff better, and a more affordable price point.
This year, CMR Surgical is rolling out enhancements in vision technology, instrumentation, and digital products to strengthen Versius’ value further.
Medtronic: Hugo continues to roll out internationally

Hugo combines wristed instruments, 3D visualization and Medtronic’s cloud-based surgical video capture and management solution, Touch Surgery Enterprise. The idea is to offer a multi-quadrant platform for a wide range of soft tissue procedures.
CEO Geoff Martha said during a November 2023 earnings call: “We expect Hugo, equipped with advanced digital capabilities, to be a meaningful growth driver for us in the years ahead.”
“We believe surgeon preference with our open console and modular design, our leading position in minimally invasive surgery and instrumentation, our connected digital ecosystem and data-enabled insights, along with our world-class surgical training program and partnerships, will meaningfully advance the low penetration of robotic surgery around the world,” he said.
Medtronic, though, has faced questions over whether Hugo will be able to compete against Intuitive’s da Vinci systems domestically. BTIG analysts checked out the Hugo system during a conference in Montreal in March 2023, and remarked in a follow-up note that they didn’t think it was different enough from existing options.
“A vast number of surgeons want choice in their robotics system, and while we’re not discounting this, we think [Medtronic’s] entrance into the U.S. will be measured,” said BTIG’s Ryan Zimmerman, Sam Durno and Iseult McMahon.
Meanwhile, Truist analysts noted in October 2023 that Medtronic’s Mazor is the first to market with a bone-cutting application, potentially a first step toward more sophisticated tissue-removing spine procedures.
Said Richard Newitter, Samuel Brodovsky, Benjamin Goldstein, and Lin Zhang at Truist: “This is the first bone-cutting application on the market and an important indication expansion as robotic technologies move to broader applicability in spine procedures beyond pedicle screw placement and working towards more sophisticated maneuvering deeper within the spine.”
Johnson & Johnson: Moving forward on Ottava
For years, Johnson & Johnson officials have only said “stay tuned” when asked about plans for the company’s Ottava soft-tissue surgical robotics system, its bid to compete with Intuitive.
In November 2023, J&J finally broke its silence, saying that it plans to submit Ottava for FDA investigational device exemption (IDE) in the second half of 2024 to initiate clinical trials.
“We are well underway to be ready for the clinical study,” Abouhalka said the next month, adding that the company plans to continue innovating in robotics “for what’s next in surgery.”
Ottava incorporates four robotic arms into a standard-size surgical table, down from the six arms shown years ago. Its unified architecture allows for an invisible design, J&J says. The robotic arms are available when needed and stowed beneath the surgical table when not.
The system also offers a “twin motion” feature, with the unified movement of the table and robotic arms. J&J designed it this way to allow surgical teams to address important clinical needs during surgery. That could include repositioning a patient without interrupting the procedure.
“With Ottava, we intend to drive simplicity and new experiences through our unique architecture,” Ottava Global President Rocco De Bernardis told MassDevice.
Meanwhile, the company’s Monarch platform and Monarch bronchoscope recently obtained regulatory approval in China, the first robot-assisted technology from Johnson & Johnson MedTech to get approved there.
In the ortho-robotic surgery space, Velys system procedures are in the thousands. J&J’s DePuy Synthes orthopedic device business boasts that Velys is a first-of-its-kind, table-mounted ortho surgery robot with an efficient design that integrates into any operating room.
Stryker: On track for new Mako applications

During Stryker’s Q4 earnings call near the end of January, company officials said the system had a record number of installations globally during the quarter. In the U.S., Stryker said 60% of its knee replacement procedures and 34% of its hip replacements were performed using Mako that year.
Mako for spine surgery is coming in the third quarter of 2024, with Mako shoulder coming near the end of the year.
In the middle of 2023, Stryker announced that it planned to boost demand for Mako-supported surgeries even more with a direct-to-patient marketing campaign in the U.S. called “Scan. Plan. Mako Can.”
Stryker officials are also excited about the potential to use AI to better analyze health data to improve surgical robotics outcomes in orthopedics.
Moon Surgical: A robotic surgical assistant
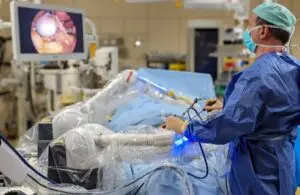
The small, adaptable system can integrate into existing clinical workflows. It features capabilities that bolster operating room efficiency and allow for alternative labor models.
Maestro’s extra set of arms offers surgeons control of two additional instruments. It provides optimal vision and access to tissue throughout the procedure, Moon Surgical says.
Moon Surgical in October 2023 saw its first U.S. clinical cases with an initial version of Maestro in Jacksonville, Florida. The next month, it announced successful surgeries in France.
The company has secured FDA 510(k) clearance and a CE mark for Maestro.
Additional Moon Surgical coverage:
Moon Surgical thinks Maestro’s light touch can win the surgical robotics arms race
Surgical robotics trends and how to accelerate adoption
Stereotaxis: A partnership with Abbott

The St. Louis-based company’s technology has treated more than 100,000 patients worldwide
Recent wins include the first patients successfully treated using the company’s Magnetic Interventional Ablation Catheter (MAGiC). MAGiC is a robotically navigated magnetic ablation catheter designed to perform minimally invasive cardiac ablation procedures. The first procedures took place at Vilnius University Hospital Santaros Klinikos in Lithuania.
Last year, Stereotaxis forged a partnership with Abbott to integrate the Abbott EnSite X EP system — a 3D heart mapping system — with Stereotaxis’ robotic magnetic navigation systems. Stereotaxis in October 2023 announced the first procedures conducted under the partnership.
Distalmotion: Seeking continued commercial expansion of Dexter

Currently in day-to-day clinical use in Europe, Dexter treats patients across complex and high-volume procedure types. That includes general surgery, gynecology and urology. Distalmotion in 2023 raised $150 million to push toward FDA approval, plus the continued acceleration of clinical experience in Europe. Zimmer Biomet and Stryker veteran Greg Roche joined as CEO near the end of the year.
Distalmotion recently partnered with Proximie, which develops an operating room operating system that provides hospitals and surgical centers access to preoperative data that can help inform patient care — plus real-time collaborative tools to record, train and deliver care.
Noah Medical: 500-case mark for Galaxy
Noah Medical recently announced the 500th use of its Galaxy surgical robotic system for robotic-assisted bronchoscopy in the U.S.
San Carlos, California–based Noah Medical designed Galaxy and its accessories to provide bronchoscopic visualization and access. These capabilities provide diagnostic and therapeutic procedures in patient airways. The system features advanced imaging technologies that provide real-time location updates for potentially cancerous lesions. Noah designed the technology to improve tool-in-lesion and diagnostic yield.
Galaxy uses proprietary integrated tomosynthesis called TiLT Technology. It enables augmented fluoroscopy with a disposable single-use bronchoscope with always-on vision, and a small, compact footprint that allows for easy integration into most bronchoscopy suites.
The company announced in April 2023 that it had raised $150 million in a Series B round.
Noah Medical CEO Jian Zhang in December 2023 shared his surgical robotics industry outlook with MassDevice’s sibling site Medical Design & Outsourcing.
Other surgical robotics companies we’re keeping an eye on at MassDevice
Procept BioRobotics is the developer of the AquaBeam robotic system, which it describes as an image-guided, heat-free robotic therapy for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. In September 2023, the San Jose, California-based company announced FDA investigational device exemption (IDE) for its Aquablation therapy. Intuitive co-founder Dr. Fred Moll has been the board chair since 2021.
Vicarious Surgical’s technology combines proprietary human-like surgical robots with 3D visualization to transport surgeons inside the patient to perform minimally invasive surgery. The Waltham, Massachusetts–based company’s technology has generated a lot of buzz in the past, but last year saw the company push back timelines for its Version 1.0 system and engage in layoffs. The company recently brought on Olympus and Flex veteran Randy Clark as president, reporting to co-founder and CEO Adam Sachs.
Momentus Surgical, formerly Memic Innovative Surgery, developed the Anovo system that featured miniature humanoid-shaped arms. CTech reported that there were layoffs at the Tel, Aviv, Israel–based company last year.
Zimmer Biomet’s Rosa surgical robotics systems for brain, hip and total knee procedures are part of the company’s overall ZBEdge digital and robotic technology suite. (Liane Teplitsky, ZB’s robotics, technology and data president, offered data integration advice and identified opportunities beyond orthopedics during an interview with MDO last year.)
Smith+Nephew offers its Cori surgical system for knee and hip surgeries. It includes a 3D intraoperative imaging system and an advanced robotic sculpting tool.
Monteris Medical is a privately held company based in Minnetonka, Minnesota, that develops MR-guided ablation systems to perform minimally invasive, robotically controlled brain surgery.
Ronovo Surgical in Shanghai unveiled its Carina robotic platform a year ago. Carina, a modular system built on proprietary technology, enables configurable robotic assistance for laparoscopic surgeries across multiple specialties.
EndoQuest Robotics — a Houston-based endoluminal surgical robotic technology developer — announced in December 2023 that it closed a C-1 preferred financing round worth $42 million in proceeds. The company designed its platform to address unmet needs in gastrointestinal and other endoluminal surgeries.
MMI (Medical Microinstruments) is the developer of the Symani surgical robotics system for microsurgery and supermicrosurgery. The Pisa, Italy–based company is led by Mark Toland, the former CEO of Corindus Vascular Robotics. The system has a CE mark in Europe. In October, MMI announced two distribution agreements covering nearly a dozen countries in the Asia Pacific region.
Levita Magnetics, in December 2023 announced the international launch of its MARS (magnetic-assisted robotic surgery) system with its placement at Hospital Luis Tisne in Santiago, Chile. The MARS system was also recently used for its first U.S. commercial cases at the Cleveland Clinic. Mountain View, California-based Levita designed the MARS platform for high-volume abdominal surgeries such as laparoscopic bariatric surgeries, cholecystectomies, and prostate and colorectal procedures.
Virtuoso Surgical has an endoscopic surgical system that it says delivers two robotically controlled, needle-sized manipulators that work from the tip of a rigid endoscope less than half the diameter of a U.S. dime. The Nashville, Tennessee-based company announced in November 2023 that it had received a $1.8 million NIH Small Business Innovation Research (SBIR) grant.
Quantum Surgical announced in September 2023 that the National Medical Products Administration (NMPA) authorized its Epione surgical robot in China. News of the regulatory nod came just weeks after Montpellier, France–based Quantum secured a CE mark expansion for Epione to treat lung tumors. A robotic-assisted percutaneous ablation system, Epione uses a robotic arm, a navigation system and a camera. It inserts a needle through the skin to the tumor and destroys it.
Medicaroid is a Japan-based surgical robotics company that is the developer of the Hinotori system, which received regulatory approval in Japan in 2020 and launched the same year. The system last year received regulatory approval in Singapore.
Triton Medical Robotics (Neptune Medical) is taking the Dynamic Rigidization technology developed at Neptune and using it to develop a fully flexible medical robot to enable doctors to reach deep into human anatomy. Neptune Medical in late 2023 announced that it had appointed Moll as its board chair.
Associate Editor Sean Whooley contributed to this article.

