A TENS device for transcutaneous electrical nerve stimulation wraps around the upper calf much like a wristwatch wraps around the wrist. Here are some of its operational secrets.
By Lee Teschler
A medical device called a Quell performs what’s called transcutaneous electrical nerve stimulation on the lower leg. This technique is said to relieve diabetic pain. None of us here are diabetic so we can’t say whether the Quell actually does what its manufacturer — Neurometrix Inc. of Waltham, Mass. — claims it can do. What we can do is describe how the Quell is supposed to work and what we found when we disassembled it.
Neurometrix filed several patents on the Quell technology. The patents include block diagrams that explain the device’s functions. So our commentary here draws upon the explanations we found in the patent as well as what we discovered when we disassembled the components.
The Quell consists of a circuit pack that fits in a fabric holster. It stimulates the leg through a stick-on set of electrodes. The electrodes have a backing of a gel-like material that firmly touches the skin. Two snaps on the electrode form both the electrical connection to the Quell electronics and the mechanical connection to the electronics housing. The housing is plastic. It holds a two-sided circuit board and the lithium-ion rechargeable battery.
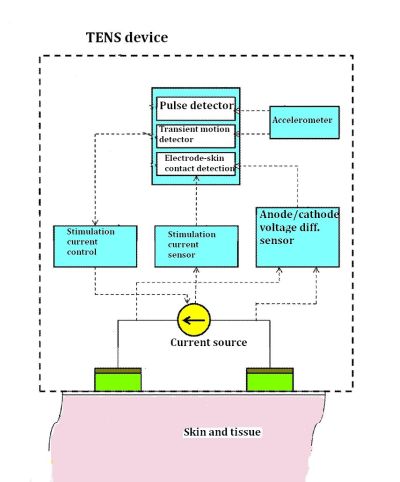
The functions of the electronics are spelled out in one of the Neurometrix patents. The electrodes use hydrogel to create a low-impedance interface between the skin and the electrode. If the portion of the electrode touching the skin peels away at all, the current density and power density rises because there’s less area of contact between the electrode and the skin. The extra power density can be painful and in extreme cases can cause thermal burns. So the unit monitors the integrity of the skin-to-electrode interface by checking the impedance of the connection continuously. If the impedance rises beyond a certain threshold, the circuit stops delivering power to the electrodes.
The hydrogel has effectively zero impedance. The electrode-to-skin impedance is generally much larger than the body’s internal tissue impedance, so dividing the anode-cathode voltage difference by the stimulation current yields the electrode-to-skin impedance.
The Quell is designed so the person using it can walk around. Of course, walking around can induce some mechanical shocks and bumps, particularly if you happen to bump into anything. But this sort of jostling can cause momentary changes in how much of the electrode touches the skin.
You don’t want the device switching off when these momentary interruptions happen. So the electronics includes an accelerometer to provide a measurement of shock impact that indicates when the electrode impedance has changed simply because the wearer is walking or has stumbled into something. According to the patent, the accelerometer is sampled at a 400 Hz rate, and processor is programmed to ignore pulses from the accelerometer that are shorter than about 50 msec.
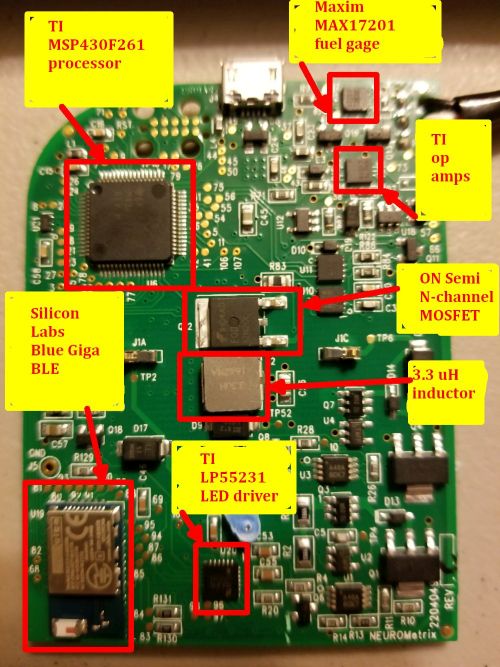
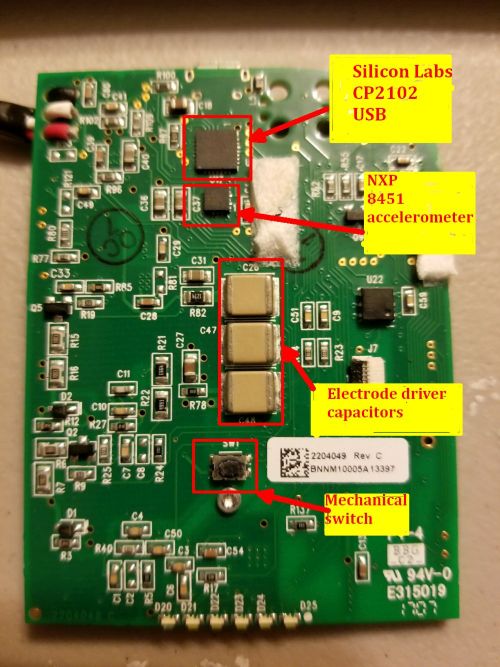
The accelerometer on the board is a three-axis 14-bit NXP device. It sits on the back of the PCB along with one other chip, a USB 2.0 controller from Silicon Labs. We should note we bought this Quell device second hand, so it’s likely that newer versions of the Quell use a more current version of a USB chip.
One interesting bit from its data sheet is that it provides access to both low-pass filtered data as well as high-pass filtered data, which is said to minimize the data analysis required for jolt detection – something we suppose would come in handy for filtering out things that happen when the wearer walks. The accelerometer can also monitor events and remain in a low-power mode during periods of inactivity.
The patent also describes how the Quell distinguishes impedance changes arising from walking or other transient conditions from those that could be more permanent. It’s all done with software flags and timers. The processor monitors the accelerometer. If the accelerometer generates a pulse, a flag gets set. The processor also monitors another flag that gets set if there’s an indication that the impedance between the electrode and the skin has risen. If the two events coincide long enough, the processor powers down the electrodes.
One Neurometrix patent shows a functional diagram of the TENS device that includes blocks for detection of pulses, transient motion, and electrode-skin contact, as well as a means of measuring and controlling the current and voltage of the two electrodes. You can be sure all these functions happen within the onboard processor. The processor on the Quell is a mixed-signal microcontroller from Texas Instruments. It is a 16-bit RISC CPU that has some features that let it wake up quickly from low-power modes.
The Quell also uses a current source, rather than a voltage source, to drive the electrodes. A Neurometrix patent indicates that driving the electrodes with pulses of current, rather than pulses of voltage, is preferable because stimulation current is independent of the impedance between the electrode and the skin, which changes while the device is in use. The voltage, of course, will change if the impedance changes.
The patent also indicates the maximum output voltage is 100 V and the maximum output current is 100 mA. The device generates a pulse pattern with intervals between the pulses that vary randomly in a 60 to 100-Hz interval. The reason for this variation is that it minimizes the tendency of the nerves in the skin to stop responding to the stimulation.
It’s pretty easy to see what the Quell uses to drive the electrodes. It is a big n-channel power MOSFET from ON Semiconductor. You normally find it in things like ac/dc power supplies. In this application, it seems over-specified because it can handle nine amps and 200 V. Though the patent talks about current pulses that are only 100 mA at most, the size of this MOSFET makes us suspect that the real Quell device uses current pulses that are a lot bigger than what the patent describes.
The MOSFET along with one sizeable 3.3 µH inductor and three ceramic capacitors seem to make up the main part of the drive circuit for the electrodes.
The processor, MOSFET, and accelerometer are the key semiconductors that handle the essential functions of the Quell. There are several other chips on the Quell that are interesting. Bluetooth communications are handled by a Silicon Labs BLE113. This is a Bluetooth Smart module that includes a host processor designed to also host end-user applications.
What’s interesting about this is that we can surmise that the software running the Quell functions was too big to fit in this Bluetooth module. Otherwise, Neurometrix would most certainly have squeezed it in and avoided the cost of the TI processor we found.
References – chips on the Quell
Silicon Labs cp2102