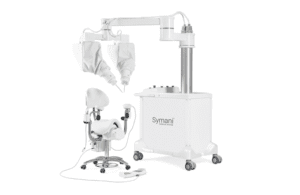
The Symani system aims to address the challenges of microsurgery with its NanoWrist instruments. These instruments help to access and suture small, delicate anatomies. That includes veins, arteries, nerves and lymphatic vessels as small as 0.3mm in diameter. It provides motion scaling and tremor reduction to allow precise micro-movements.
Pisa, Italy-based MMI’s Symani received CE mark in 2019, and the company continues to work on U.S. commercialization. The first-of-its-kind robotic technology enables expanded adoptions for patients in need of sot tissue open surgical procedures.
Now, since its first-in-human cases in October 2020, Symani surpasses the 500-procedure milestone. MMI says it rapidly increases patient access to surgical treatment options as surgeons become more comfortable with the technology.
Dr. Sinikka Suominen of the HUS Helsinki University Hospital in Finland performed a lymphatic repair to mark the 500th case. Of the 500 procedures to date, roughly 75% have been free flap surgeries, with 16% being lymphatic repairs. The remaining 9% include peripheral nerve repairs and extremity replantation surgeries.
Suominen noted that the Helsinki hospital uses Symani across multiple procedure types. The doctor says it’s demonstrated “notable changes in the accuracy, stability and reliability” in suture placement for lymphatic procedures.
“We believe that open surgery is long overdue for technological advancement. This major milestone for the Symani Surgical System is a testament to how robotics can elevate the standard of care by pushing the boundaries of complexity,” said Mark Toland, CEO of MMI. “People with hard-to-treat conditions deserve options that can give them a better quality of life, and we hope that by expanding access to microsurgical and supermicrosurgical procedures, we’re able to drive that initiative forward.”

