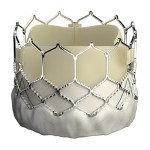
 Edwards Lifesciences (NYSE:EW) said today that the Japanese Ministry of Health, Labor & Welfare approved its Sapien 3 transcatheter aortic valve replacement.
Edwards Lifesciences (NYSE:EW) said today that the Japanese Ministry of Health, Labor & Welfare approved its Sapien 3 transcatheter aortic valve replacement.
Irvine, Calif.-based Edwards said it expects to win reimbursement approval in Japan during the 2nd quarter and launch “immediately thereafter” with a full rollout complete by the end of the year.
“We are pleased to be able to offer Japanese clinicians and their patients access to the latest TAVR therapy with a smaller-profile transfemoral delivery system that will allow for the treatment of many patients with small or difficult-to-navigate vasculature,” vice president Dr. Huimin Wang said in prepared remarks. “We appreciate the MHLW’s progressive decision-making in allowing this device to be available to Japanese patients in a timely manner, bringing Japan alongside the U.S. and Europe.”
In January, the FDA approved a new trial of the Sapien 3 valve for low-risk patients. The device was approved last year by the federal safety watchdog for high-risk patients.
The Sapien 3 valve, designed in part to address some of the concerns about leakage in earlier generations of valves, has been on the European market since approval in January 2014. The Sapien 3 is Edwards’s 3rd-generation Sapien heart valve, originally approved by the FDA in 2011.

