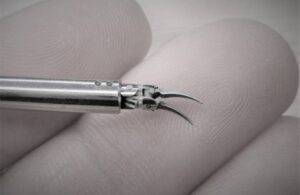
The clearance covers soft tissue manipulation to perform microsurgery. This technique involves reconnecting tiny vessels to restore blood flow or redirect fluid during reconstruction or repair.
FDA authorization makes Symani the only commercially available platform in the U.S. for reconstructive microsurgery, according to a news release. Medical Microinstruments believes its technology could open the field of microsurgery to new surgeons by quickly developing their skills. Additionally, it could empower skilled microsurgeons to expand into “supermicrosurgery.”
The company says this could create a novel category of treatments that human hands can’t perform without robotic assistance.
“The U.S. is facing a potentially dire shortage of physicians, and that shortage acutely impacts specialized fields of medicine, such as microsurgery,” said Mark Toland, CEO of Medical Microinstruments. “With the authorization from the FDA, our technology will expand its reach to pioneering hospitals in the U.S. It will help those hospitals grow their open surgical programs, expand the number of physicians who can perform these highly complicated procedures, and increase patient access to the most advanced techniques for surgeries in complex disease states, such as lymphedema. Our system will continue to provoke surgeons to challenge their definitions of ‘treatable’ and ‘untreatable’ and empower them to solve cases that have historically been too difficult to treat.”
Pisa, Italy-based Medical Microinstruments’ Symani first received CE mark in 2019. The first-of-its-kind robotic technology enables expanded adoptions for patients in need of soft tissue open surgical procedures.
Investors see “considerable opportunity” for rapid growth with the company, culminating in a $110 million Series C round raised in February. The company has brought in more than $200 million in funding to date, including a $75 million Series B in 2022.
More about the new opportunity for Medical Microinstruments
Symani provides advanced solutions for a range of open surgeries. That includes post-mastectomy breast cancer reconstruction, extremity reconstruction using free tissue transfer and lymphatic system repair.
The company’s tiny NanoWrist instruments help to access and suture small, delicate anatomies. That includes veins, arteries, nerves and lymphatic vessels as small as 0.3mm in diameter. It provides motion scaling and tremor reduction to allow precise micro-movements. The articulated wrist features seven degrees of freedom that match the human wrist, tremor filtration and motion scaling.
Surgeons have used Symani in nearly 1,000 clinical cases in Europe, plus thousands more in preclinical situations around the world. It already has availability for commercial use in Europe and parts of Asia Pacific.
Medical Microinstruments plans to immediately launch the technology in the U.S.
“By making open surgery less invasive and more precise, we can treat more conditions and offer robotic-assisted surgical options to patients that simply do not exist today,” said Dr. L. Scott Levin, co-CMO of Medical Microinstruments. “Within the next five years, this expanded portfolio of addressable open surgical procedures is expected to exceed the number of eligible laparoscopic, or minimally invasive, procedures that leverage robotic assistance. The authorization from the FDA helps to solve a critical unmet need and will help surgeons perform a new category of complex open surgeries enabled by transformative technology.”

