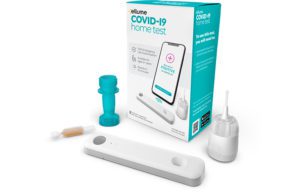
The Class I recall had only been in the hundreds of thousands of kits when first announced in October.
The Australian company has stressed that the reliability of negative results is unaffected by the problem.
“Ellume has investigated the issue, identified the root cause, implemented additional controls, and we are already producing and shipping new product to the U.S.,” a company representative told the New York Times.
FDA, however, still considers the recall serious because false positives could delay diagnosis and treatment for whatever is actually ailing people — and could cause them the undergo unneeded COVID-19 therapies.
People with false positives could also unnecessarily isolate themselves or miss work.
FDA said it has received 35 reports of false-positive results, with no deaths reported.
Ellume has alerted retailers to remove affected products from shelves and stop sales. The company also has an online form for people who have already purchased the tests to request replacements.

