
Analysts at Citron Research published their latest scathing report on embattled Intuitive Surgical (NSDQ:ISRG), citing a surge in reported adverse events that the medical device maker says is a flurry of filings, not a in increase in incidents.
Intuitive Surgical maintained that a closer look at the FDA’s MAUDE adverse events database shows a decrease in patient injuries and deaths amid an increase in filed reports over the last 3 years, a company spokesperson told MassDevice.com today.
"Any assessment of safety trends based on Reporting Dates rather than Event Dates in the MAUDE database will be fundamentally flawed and misleading, and is likely to seriously misrepresent the true performance of a device," according to a company statement.
Citron analysts chided the company as well as other analysts for underestimating the concerns that have dragged down ISRG shares by about a third since this time last year. Intuitive Surgical in recent months has been the target of an FDA probe into surgical complication rates, an American Congress of Obstetricians & Gynecologists statement questioning the benefits of da Vinci surgery, a high-profile patient injury lawsuit painting the company as an aggressor, a surgical instrument crack warning and a shareholder class action lawsuit against the company and its leadership.
Where other analysts have pressed the company to "do something about the negative press," Citron analysts warned that the harsh headlines were a symptom of a larger problem, warning that Intuitive Surgical is "not investable til it gets its house in order." Citron further called on the company to take responsibility and address what it called a "surprising" surge in adverse events and deaths reported in 2013.
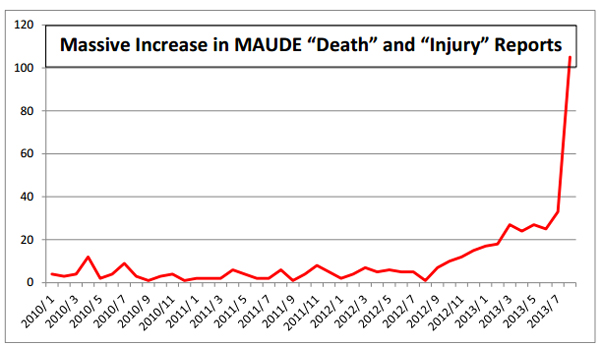
Citron’s graph of 2013 MAUDE reports including deaths and injuries associated with the da Vinci system.
"In the 1st 8 months of 2013, 2332 Adverse Event records were posted – compare to 4603 records posted in the entire 12 year period since the 1st Adverse Event tracking for da Vinci appeared in MAUDE in 2000," Citron wrote. "It is the opinion of Citron that the only reason there is not a national outcry is because the da Vinci robot has yet to kill or injure ‘the right person’ – like the next of kin of a congress member or a celebrity."
Intuitive Surgical spokeswoman Angela Wonson denied that there was a true increase in da Vinci incidents, saying that Citron examined the dates the events were reported, rather than when they actually occurred.
"Understanding this Reporting Date/Event Date disconnect is particularly important if a quantity of adverse event notifications are received in a short timeframe – e.g., if a news story, Internet posting, or advertising or legal campaign generates a sudden flurry of claims," according to an Intuitive Surgical statement on the Citron report. "In such a situation, a batch of reports covering the adverse events would be submitted to the FDA, and the resulting MAUDE data would exhibit a surge in entries clustered around a recent reporting date. However, the underlying events may have occurred over a much longer period of time, and therefore the data would not be indicative of a sudden increase in adverse events."
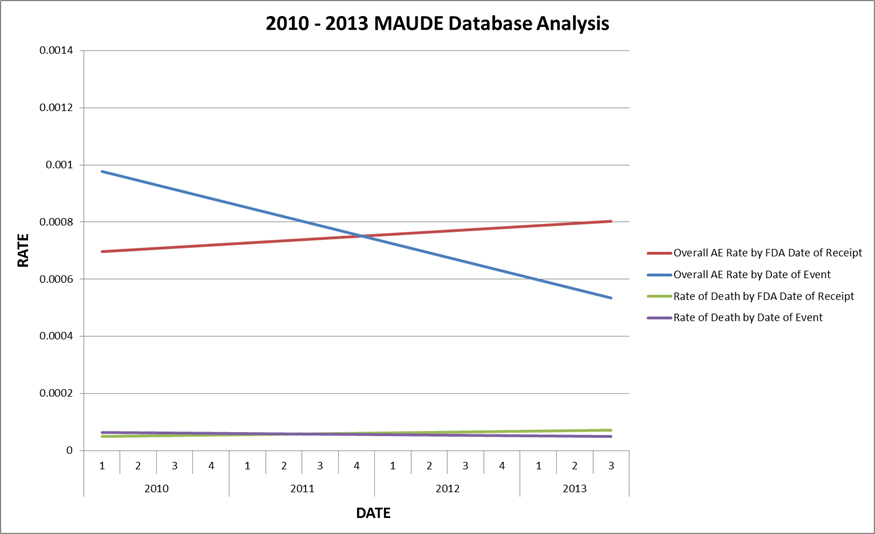
Intuitive Surgical’s analysis of MAUDE reports associated with its da Vinci system.
California-based Intuitive Surgical conducted its own analysis of the MAUDE database, which both regulators and researchers warn isn’t a very credible means of tracking true incident rates or comparing individual technologies against others.
An analysis of reports dating back to 2010 suggests that actual incidents have decreased over the last few years, while reporting has increased, the company concluded.
"Because a medical device manufacturer can only report an adverse event to the FDA after it is notified of the event, and because that notification may not be received until months or even years from the time of the event, a MAUDE database entry with a relatively recent reporting date may be associated with an adverse event that occurred much farther in the past," Intuitive Surgical said. "Thus, any assessment of safety trends based on Reporting Dates rather than Event Dates in the MAUDE database will be fundamentally flawed and misleading, and is likely to seriously misrepresent the true performance of a device."
Intuitive Surgical’s share price lost 25.3% this year alone as the company struggled with a series of studies finding robotic surgery equally safe and effective but more expensive than laparoscopy. Investors, who sent ISRG shares to an all-time high of $585.82 apiece in April 2012, have carved some 36.6% from the stock since then.
Prices tumbled 11.1% in a single day last February, to $509.33 per share, after a report in the Journal of the American Medical Assn. also questioned the value of hysterectomies using robotic surgery. In March, the president of the American College of Obstetricians & Gynecologists, Dr. James Breeden, criticized robotic surgery hysterectomies.
A study published just last month found that robotic surgeries may be under-reported and therefore less safe than they appear, a conclusion Intuitive called "misleading." Studies published last year raised similar concerns regarding robotic surgery prostatectomies and robotic surgeries to treat endometrial cancer.

