Go directly to MassDevice. Having trouble reading this email? View this newsletter in your browser here.
Not interested? Unsubscribe.
The MassDevice Weekly Roundup brings you the latest medical device news and information.
The MassDevice Checkup
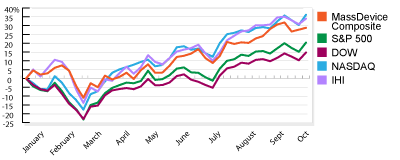 Our weekly checkup takes the temperature of the public medical device companies in the three largest markets in the U.S.: Massachusetts, Minnesota and California. In addition to the indices for each state, which track the overall movement of the sector, we compare Monday morning’s opening share prices with their value when the markets close Friday afternoon and calculate the biggest gainers and losers. Read More
Our weekly checkup takes the temperature of the public medical device companies in the three largest markets in the U.S.: Massachusetts, Minnesota and California. In addition to the indices for each state, which track the overall movement of the sector, we compare Monday morning’s opening share prices with their value when the markets close Friday afternoon and calculate the biggest gainers and losers. Read More ![]()
![]()
News
Systagenix Wound Management settles in Quincy, Mass.
The former wound care products business of J&J subsidiary Ethicon taps Quincy, Mass., for its Americas HQ; names 13-member medical advisory board. Read More ![]()
SeraCare to sell former HQ, pays off $10 million credit line
The Milford, Mass.-based life sciences service provider inks a deal to sell its former base in West Bridgewater, Mass., for $1.35 million and closes its revolving credit facility with GE Capital. Read More ![]()
Medical device sales at J&J beat pharma revenues for the second quarter running
Johnson & Johnson’s DePuy and orthopedic divisions post third-quarter gains, but its Cordis Corp. subsidiary is down on weak stent sales as the healthcare products conglomerate reports a modest earnings uptick. Read More ![]()
Judge tosses former IST CEO Schorer from lawsuit
A federal judge dismissed charges against former Innovative Spinal Technologies CEO Scott Schorer in a fraud and breach of contract case. Read More ![]()
Boston Scientific buys the distribution rights to Pluromed’s BackStop
The Natick, Mass.-based medical devices giant will distribute the Woburn firm’s kidney stone treatment worldwide. Read More ![]()
Immuneering heads to the Dog Patch
The company, which is working on a system to help oncologists predict whether cancer patients will respond to certain treatments, wins a slot in Polaris Venture Partners’ Cambridge, Mass., startup incubator. Read More ![]()
House approves FDA budget boost
The Fiscal Year 2010 Agriculture Appropriations Act contains provisions for a $34 million boost to the Food & Drug Administration’s medical device division, but the increase is less than the 24 percent boost approved by the House earlier this year. Read More ![]()
Dana Farber licenses “cold” PCR technology to Transgenomic
The Omaha-based biotech firm will use the cancer institute’s cold polymerase chain reaction technology to develop highly sensitive, blood-based diagnostics to detect genetic mutations that might cause cancer. Read More ![]()
Covidien escapes patent suit filed by Thermal Scalpel with licensing deal
The Newport Beach, Calif.-based firm drops Covidien and its corporate predecessor, Tyco Healthcare, from a lawsuit after cutting a deal to license the technology. Read More ![]()
Helicos BioSciences wins $2.9 million grant from National Human Genome Research Institute
The Cambridge, Mass.-based maker of genetic sequencing equipment gets a piece of the stimulus money. Read More ![]()
Massachusetts healthcare payment reform could be a boon for the HIT industry
Stakeholders at a public hearing call electronic medical records critical to implementing a global payment system and warn of dire consequences from inaction on payment reform. Read More ![]()
EU green-lights Medtronic’s Captivia
The Minneapolis-based devices giant lands CE Mark approval from the European Union for its thoracic stent graft delivery system for treating aortic aneurysms. Read More ![]()
 CBO: Baucus bill shaves deficit by $81 billion
CBO: Baucus bill shaves deficit by $81 billion
Healthcare reform legislation out of the Senate Finance Committee would pare $81 billion over 10 years from the federal deficit, according to a preliminary estimate from the Congressional Budget Office. Read More ![]()
Bruker names three directors for new energy unit
Two outside executives and an academic fill new seats on Bruker Energy and Supercon’s governing panel. Read More ![]()
Analyst: Cosmetic procedure numbers may be stabilizing, but the laser market is still a cause for concern
Leerink Swann’s MEDACorp survey of 100 dermatologists and plastic surgeons indicates that the Botox and breast implant markets could grow in the next six months, despite another down quarter, but will recovery leave the laser market behind? Read More ![]()
LiveData gets Phase II SBIR grant for operating room dashboard
Cambridge-based company gets $750,000 to work on plug and play integration for operating room dashboard with CIMIT. Read More ![]()
Healthcare industries’ tab for reform: $121 billion
Healthcare-related industries would be forced to pony up more than $12 billion a year, collectively, under the healthcare reform legislation being considered in Washington. Read More ![]()
CeQur taps J&J veteran Eric Milledge for board chairmanship
The Swiss insulin delivery company, which has offices in Marlborough, Mass., taps the 34-year Johnson & Johnson veteran to chair its board. Read More ![]()
Biosphere touts new embolization study
The Rockland, Mass.-based firm cites trial data showing that its uterine artery embolization treatment outperforms laparoscopic occlusion of uterine arteries in treating fibroids. Read More ![]()
MIT’s Deshpande Center doles out $600,000 in grants
The tech incubator awards grants to eight MIT research teams to develop early-stage technologies. Read More ![]()
Ex-Biopure cancer faker Howard Richman gets 3 years
A former executive for the defunct Cambridge, Mass.-based blood substitute maker gets a 36-month sentence and a $50,000 fine for faking cancer to avoid an SEC lawsuit. Read More ![]()
Report: Senate delays vote on healthcare reform
The Senate Finance Committee will wait for a report from the Congressional Budget Office on the cost of its healthcare reform proposal before its final vote, pushing the full Senate’s debate back at least a week. Read More ![]()
Mpathy touts study of its Omnisure SUI sling
The 61-patient trial showed a 92 percent cure rate after implantation of the device to treat female stress urinary incontinence. Read More ![]()
HeartWare wins FDA approval to expand clinical trial
The Advance bridge-to-transplant trial of the Framingham, Mass.-based cardiac pump maker’s ventricular assist system will grow from 28 to 40 sites. Read More ![]()
FDA ups post-market scrutiny of spinal implant systems
The Food & Drug Administration is requiring manufacturers of dynamic stabilization systems to run post-market studies on their safety and efficacy after implantation. Read More ![]()
Boston Scientific lands FDA nod, opens new defibrillator trial
The Food & Drug Administration clears the latest version of the WallFlex biliary stent; BSX begins enrollment in defibrillator trial. Read More ![]()
Athenahealth gets anodyzed
The Watertown, Mass.-based electronic medical records provider agrees to acquire Saas provider Anodyne Health Partners for up to $30 million. Read More ![]()
Inverness says test can identify acute kidney injury
The Waltham, Mass.-based diagnostics giant says a new study in the Journal of Critical Care shows promising results for its Triage NGAL Test. Read More ![]()
![]()
Sponsored Blog
 Marketing Mavens: A gurney’s eye view of your customers
Marketing Mavens: A gurney’s eye view of your customers
Seeing medical device customers in their natural habitat can be enlightening for a marketer. Read More ![]()
Jobs
The MassDevice Career Connector: Open jobs in the medical device industry
Our jobs board has hundreds of open positions at medical device makers around the country. Read More ![]()
![]()
Features
Mass Device Q&A: Campbell Rogers
Cordis Corp.’s chief scientific officer on the move from bedside to boardroom, and what Cordis has in its development pipeline. Read More ![]()
Mass Device Q&A: Dave Kroll
The founder of Kroll Associates on why distance doesn’t matter and the role small, focused firms will play in the expansive and varied medical devices field. Read More ![]()
![]()
Blogs
MassDevice blog: So much for sunshine
Orthopedists left out more than 20 percent of the payments they landed from medical device makers in their research presentations, according to a study in the New England Journal of Medicine, bolstering arguments for so-called “sunshine laws” mandating disclosure of industry payments to physicians. Read More ![]()
Weekly Wireless Roundup: Medtronic seeks wireless health partners
Medtronic sees potential in partnering with other companies for wireless health; White House hints at Text4Baby launch next week; 81% of physicians will use smartphones by 2012; and Google Health inks deals with two insurers. Read More ![]()
MedGadget’s MedTech Monday: An inside look at a Philips Respironics plant
Our friends over at MedGadget.com got an inside look at Philips Respironics’ new plant in New Kensington, Pa.; a project to test RFID interaction with medical devices; getting a grip on glucose monitoring; and the RAY portable X-ray device. Read More ![]()
MassDevice blog: Could Wal-Mart and BlackBerry be the future of medicine?
Biotechnology analyst Steve Burrill says biotech companies should be thinking about the consumer if they want to meet the challenges of medicine in 2020. Read More ![]()
Reimbursement Row: Is data integrity the Achilles’ heel of healthcare reform?
Not at all. In fact, it just might be a compelling rationale for the public option. Read More ![]()
Dollars & Spence: Does D.C. understand VC?
Maybe, now that alternatives to treating venture capital the same as hedge funds are proposed in Washington. Read More ![]()
![]()
Upcoming Events
Featured Events
Oct. 21, Newton, Mass. — Medical Development Group networking mixer, “Post-Approval Studies – Maximizing the Value of Clinical Experience”
“This program will provide an overview of clinical trials at various stages following the submission of an application for a regulatory clearance including trial planning, control, identification of data that has to be reported to the FDA and possible implications for the commercial use of the device.”
 Oct. 27-28, Waltham, Mass. — Medical Product Outsourcing Symposium East
Oct. 27-28, Waltham, Mass. — Medical Product Outsourcing Symposium East
MassDevice users can save $400 on conference registration by using the code “MassDev”
“The practice of outsourcing continues to present challenges to medical devices manufacturers. This event is a high-level educational and networking program, designed to offer relevant information for solving business-critical issues in an environment that fosters interaction and industry development.”
Oct. 27-28, Boston, Mass. — CIMIT Innovation Congress 2009
MassDevice users can save 10 percent on conference registration by using the code “MO-DEV”
“Accelerating Healthcare Solutions Through Technology: The CIMIT Innovation Congress is the premier event catalyzing innovations in medical device technology. It’s the only place where academia, government, industry, and the military convene to learn and network with national leaders who are shaping tomorrow’s healthcare solutions.”
Other events
Oct. 15, Boston — Innovation Day at the Statehouse
Oct. 19-20, Lowell, Mass. — U.S./Ireland Emerging Technologies Conference
Oct. 22-23, New York City — Gel Health
Oct. 23, Cambridge, Mass. — Healthcare and the Cloud
Oct. 26-27, Waltham, Mass. — New England-Israel Life Sciences Summit
Oct. 27, online — Marsh Risk Consulting’s free webinar, “Monetizing Intellectual Property: Selling or Licensing your IP”
Oct. 28th, Waltham — The Capital Network presents “Networking for Fundraising Success”
Want to list your event on our weekly newsletter? Contact us at editor@massdevice.com.
![]()
If you would like to subscribe to this newsletter, please visit our member registration page. ![]()
© 2009 Massachusetts Medical Devices Journal LLC and its licensors. All rights reserved. The material on this site may not be reproduced, distributed, transmitted, cached or otherwise used, except with the prior written permission of MMDJ.



